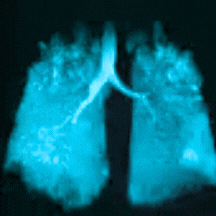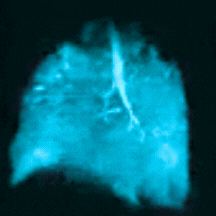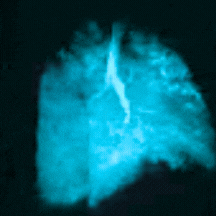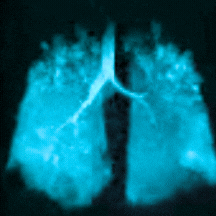
Bringing Xenon MRI into the radiology department provides substantial opportunities to enhance patient care and generate revenue for the institution. In particular, the recent FDA-approved label expansion (update to include children 6 years and older) now opens up this non-invasive, non-radioactive diagnostic tool to more patients in need of a more precise method to assess and quantify regional lung function.
In this webinar, you will hear from experts at Cincinnati Children’s Hospital Medical Center, the first institution to pioneer clinical imaging with Xenon MRI, as they share how to coordinate across departments, schedule patients efficiently, and develop standardized radiology analysis and interpretations to support the care of children with debilitating lung disease.
Please see full Prescribing Information.
Important Safety Information
XENOVIEW®, prepared from the Xenon Xe 129 Gas Blend, is a hyperpolarized contrast agent indicated for use with magnetic resonance imaging (MRI) for evaluation of lung ventilation in adults and pediatric patients aged 6 years and older.
XENOVIEW® has not been evaluated for use with lung perfusion imaging.
None.
Risk of Decreased Image Quality from Supplemental Oxygen: Supplemental oxygen administered simultaneously with XENOVIEW® inhalation can cause degradation of image quality. For patients on supplemental oxygen, withhold oxygen inhalation for two breaths prior to XENOVIEW® inhalation, and resume oxygen inhalation immediately following the imaging breath hold.
Risk of Transient Hypoxia: Inhalation of an anoxic gas such as XENOVIEW® may cause transient hypoxemia in susceptible patients. Monitor all patients for oxygen saturation and symptoms of hypoxemia and treat as clinically indicated.
Adverse Reactions in Adult Patients: The adverse reactions (> one patient) in efficacy trials were oropharyngeal pain, headache, and dizziness.
Adverse Reactions in Pediatric Patients: In published literature in pediatric patients aged 6 to 18 years, the following transient adverse reactions were reported: blood oxygen desaturation, heart rate elevation, numbness, tingling, dizziness, and euphoria. In at least one published study of pediatric patients aged 6 to 18 years, transient decrease in SpO2% and transient increase in heart rate were reported following hyperpolarized xenon Xe 129 administration. XENOVIEW® is not approved for use in pediatric patients less than 12 years of age.
Please see full Important Safety Information
Copyright © 2025 POLAREAN. All rights reserved.
Fill up the form and we'll send you the recording of our previous webinar.